Your cart is currently empty!
How to minimize uncertainty and maximize efficiency of your ADC development experiments
Antibody-drug conjugates (ADCs) are a novel type of targeted therapy used for the treatment of cancer. By combining cytotoxins with the targeting capabilities of monoclonal antibodies, ADCs can be used to deliver and release highly-potent cancer killing drugs directly within tumor cells. The high specificity and targeted delivery afforded by ADCs is making them favorable over conventional chemotherapy approaches within the field of oncology.
ADC development process: high-throughput prototyping during the preclinical phase is crucial yet challenging with traditional antibody labeling technology
Prior to launching an effective antibody-drug conjugate for clinical use, years of rigorous in-vitro tests must be conducted to effectively hone in on the optimal antibody-drug pair(s). Given the high costs and time dedicated towards in vivo testing and clinical trials, the preclinical “prototyping” phase of ADCs is crucial to quickly yet effectively identify the top antibody-drug conjugate combination that could yield promising results in further trials. Successful ADC development is dependent on several factors including the specificity of the antibody, potency of the drug, drug-antibody ratio (known as the DAR), linker stability, and the drug distribution on the antibody. All these factors will affect the safety, efficiency, and delivery of ADCs to target cells. Therefore, the ability to tightly control the labeling process will greatly influence the success of ADC development. It is absolutely essential for researchers to use a drug conjugation method that both minimizes uncertainty and maximizes efficiency.
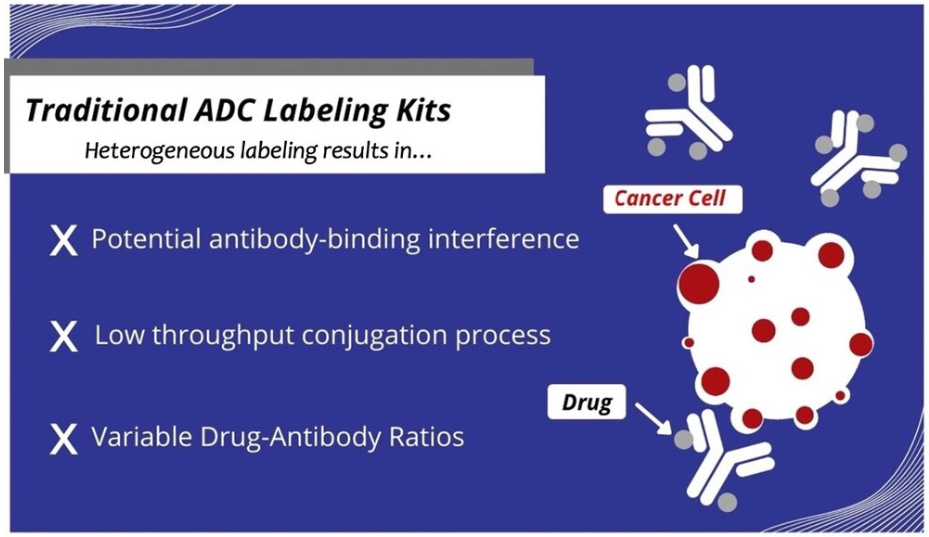
With existing antibody labeling technology, two major problems arise during ADC prototyping: 1) the high-throughput capability is limited and 2) uncertainties arise from non-uniform antibody-drug conjugates.
Existing antibody-labeling techniques are not suited for high-throughput ADC prototyping
When trying to effectively kill tumor cells for a specific cancer, there are a variety of cellular targets one could choose that might lead to a breakthrough treatment. Thus, it is optimal to test multiple antibodies during the early ADC development stage in efforts to assess the impact on tumor-killing from binding specific targets with their corresponding antibodies. Furthermore, antibodies can vary in their binding affinity or bind to different epitopes on the same target, both of which can have an effect on ADC efficacy. With hundreds or potentially thousands of targets to choose from, it is often necessary to run many assays in parallel with different antibodies in an ADC format to determine which is best. The drug payload is another crucial component of the ADC: this cytotoxic payload is chaperoned to the tumor cells by the antibody, ideally leading to high potency killing of the tumor cell. It is important to test different payloads with different antibodies to determine which antibody-drug combination yields the highest tumor cell killing. Given the numerous combinations possible for one ADC candidate, it is essential to have a high-throughput experimental method in which many different conditions can be tested in parallel, allowing researchers to quickly sort out the best antibody-drug combinations for further testing.
The antibody labeling method is a crucial part of this process: researchers must have a quick and effective way of attaching their antibody to their drug. Unfortunately with existing labeling technologies, purification of excess drug and linker is often a necessary step prior to testing the ADC in a cell-killing assay. This extra purification step or steps add additional time, reagents, and costs to the overall experimental plan, which can greatly reduce the high-throughput capability and efficiency of ADC prototyping. Furthermore, traditional antibody labeling techniques often require a minimum of 50-100 µg of antibody to be labeled per conjugation reaction. This lower bound to the minimum amount of antibody that can be labeled with the drug results in a limit of how many different antibodies can be tested with one batch of drugs and labeling reagents, which in turn limits throughput.
Key takeaway: Antibody linker technology should enable high-throughput ADC prototyping, allowing rapid plug and play conjugations with various antibodies and toxic payloads.
Existing antibody labeling techniques do not yield uniform ADCs, leading to experimental uncertainty
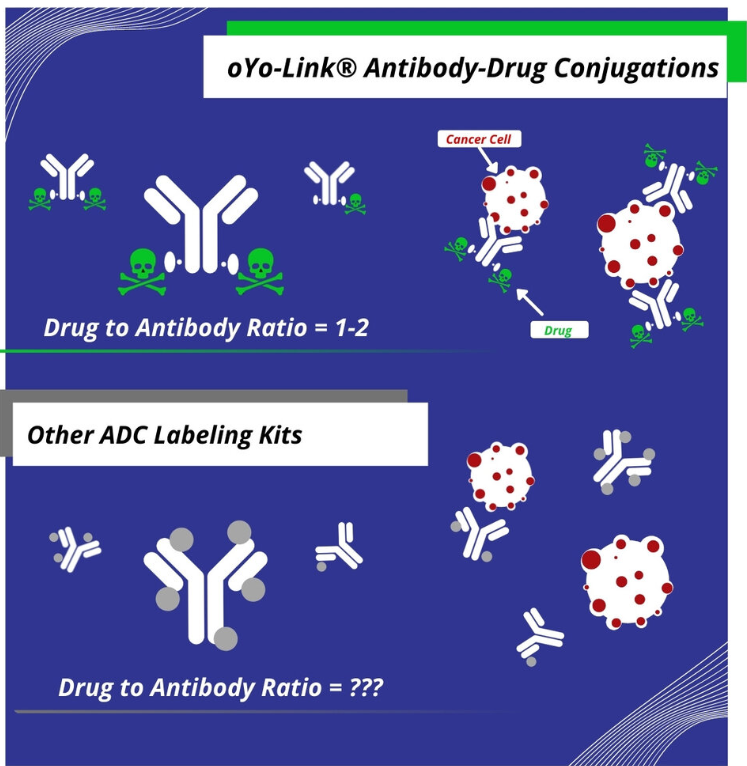
In addition to limited high-throughput capability, existing antibody labeling technologies can also lead to highly heterogeneous conjugates and variability in the DAR for different antibodies. This is due to the non-site-specific labeling nature of these common chemical-based antibody conjugation techniques, which typically attach drugs to the lysines on the antibody in a random and uncontrolled manner. The resulting non-specific conjugates can lead to blocking of the antigen-binding region on the antibody, leading to reduced effectiveness of the ADC. Furthermore, the number and location of drugs attached to each antibody can vary significantly. As aforementioned, the drug-to-antibody ratio (DAR) is a crucial parameter in ADC development. During ADC prototyping, it is important to minimize the variability of the DAR to isolate the effects of the antibody-drug combination on efficacy. If the DAR is too widespread, it could prove difficult to attribute certain results of an experiment to the antibody selected, drug selected, or other parameters changed.
Key takeaway: Antibody labeling methods should yield uniform ADCs with a well-defined DAR so that experimental uncertainty can be minimized during prototyping.
Introducing oYo-Link® antibody-drug conjugation reagents
Convenient site-specific conjugations yielding homogeneous ADCs for rapid prototyping
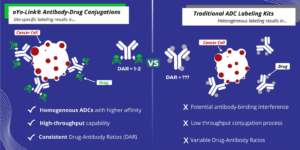
AlphaThera’s oYo-Link labeling reagents enable rapid prototyping of ADC candidates by allowing site specific conjugations of drugs to antibodies for use in cancer research applications including targeted cell death and high-throughput screening with cell killing assays. oYo-Link reagents consist of low molecular weight (~8 kDa), high-affinity antibody-binding domains that possess a photo-crosslinker within their Fc-binding site. Upon illumination with non-damaging Black-light, oYo-Link forms a covalent bond with the antibody. The resulting conjugates guarantee attachment of 1-2 drugs per antibody specifically in the Fc-constant region.
oYo-Link provides 3 main advantages for ADC development & prototyping:
-
Uniform, site-specific conjugates with minimal uncertainty: The site-specific labeling enabled by oYo-Link provides precise control over conjugation sites resulting in a homogeneous ADC. Site-specifically labeling attaches the drugs to the Fc-region every time, instilling confidence that the antibody binding region is free. Furthermore, these conjugates have a well-defined DAR of 1-2 drugs per antibody, yielding consistent ADCs with predictable efficacy and toxicity profiles.
-
No Purification of excess drug required: A unique aspect of the oYo-Link Drug reagents is that when oYo-Link is bound to the drug, the free oYo-Link-Drug that is unbound to the antibodies will not be toxic to cells. Thus, no purification is needed following antibody conjugations – you can proceed directly to your cell killing assays. This greatly reduces the time involved with ADC prototyping and affords more flexibility with high-throughput experiments.
-
Use less antibody: oYo-Link can label as little as 1 µg of antibody at a time with oYo-Link ADC reagents, allowing researchers to conduct many more experiments with one batch of antibodies and drugs. This reduces costs and enables more high-throughput screening.
In addition to a precise DAR as shown on the left, oYo-Link reagents provide several convenience advantages when prototyping ADCs. These include:
Robust Antibody & Buffer Compatibility
Works with nearly all off-the-shelf antibody species and subclasses. Reactions are insensitive to all common buffers, a wide range of pH values, and the presence of storage proteins (e.g. BSA).
-
Rapid labeling reaction
oYo-Link ADC reactions enable extremely quick antibody to drug conjugation, requiring less than 30 seconds hands-on time, with ADCs ready in 2 hours total. Furthermore the labeling is highly efficient, resulting in homogeneous products with one-to-two drugs per antibody.
-
Easily confirm that your Antibody is labeled with SDS-PAGE: oYo-Link consists of a small (8 kDa) photoreactive antibody-binding domain that site-specifically and covalently labels the heavy chain of antibodies upon Illumination with a black light. Successful photocrosslinking leads to a slight increase in the molecular weight of the antibody heavy chain, which can be easily detected by a noticeable upward shift on a gel.
A precise DAR is key to understanding results of cell-killing assays when testing your ADCs. oYo-Link assures 1-2 drugs per antibody, removing uncertainty when analyzing your data.
The oYo-Link range of drug conjugation products includes VcMMAE, VcMMAF, DM1 and DM4, and enables conjugation to the majority of off-the-shelf antibodies.